A Complete End-to-End Prescription Drug Monitoring Program Reporting & Management System
With over 10 years of experience, our team has the knowledge and expertise to address the complexities and challenges associated with state prescription drug monitoring program (PMP) reporting. Using our simple and streamlined process will allow you to boost productivity and make resources available for other tasks.
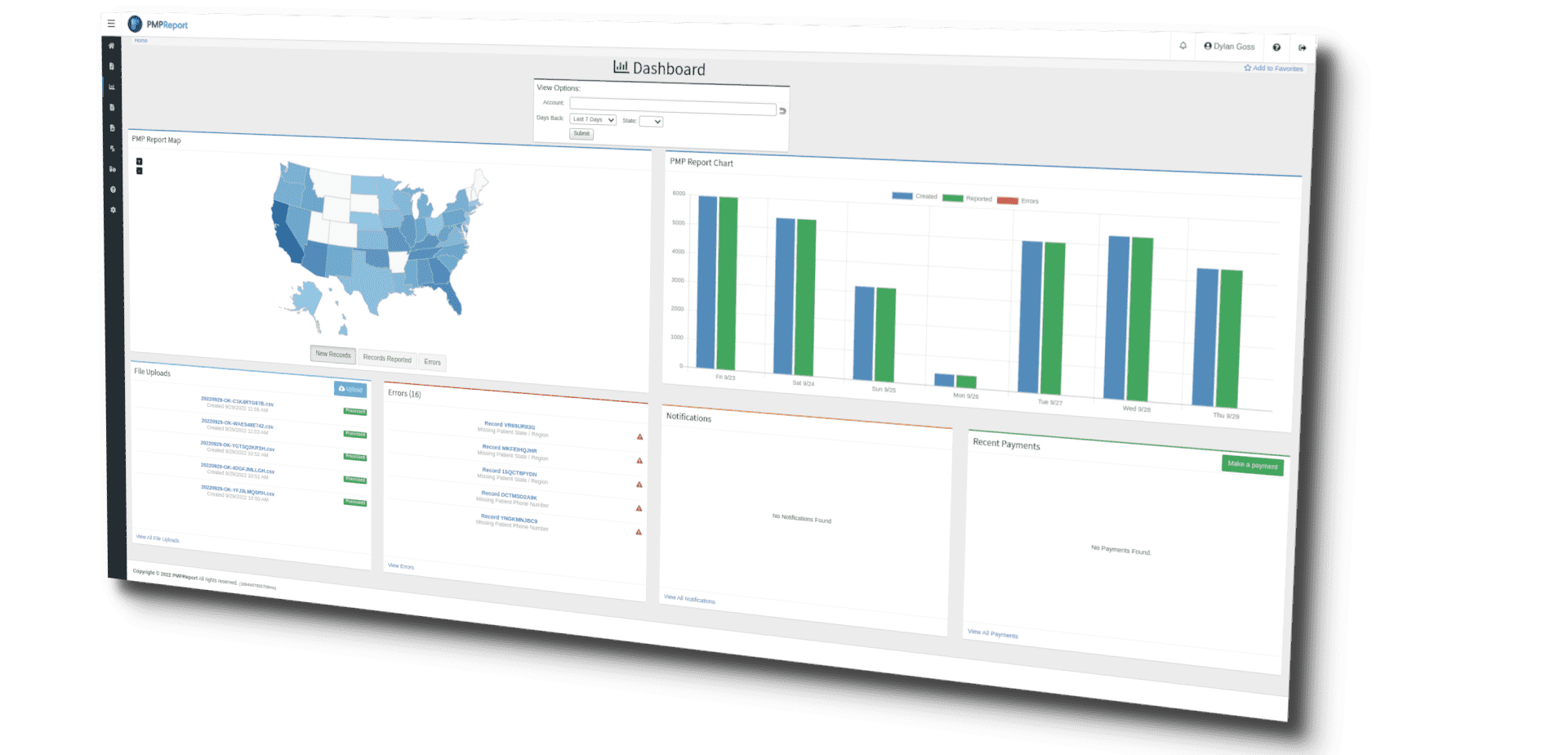
State PMP agencies can vary widely on such things as drug monitoring lists, report formats, reporting frequencies, and laws for noncompliance. Our complete PMP management system will give you complete confidence in compliance with PMP requirements. PMPReport is a complete PMP Reporting solution regardless of whether you report for a single location or hundreds in a single state or multiple. Our straightforward pricing allows for reduced overhead and streamlined budgeting.